Open Access Article: Lower the risk of chemotherapy-induced peripheral neuropathy (CIPN)
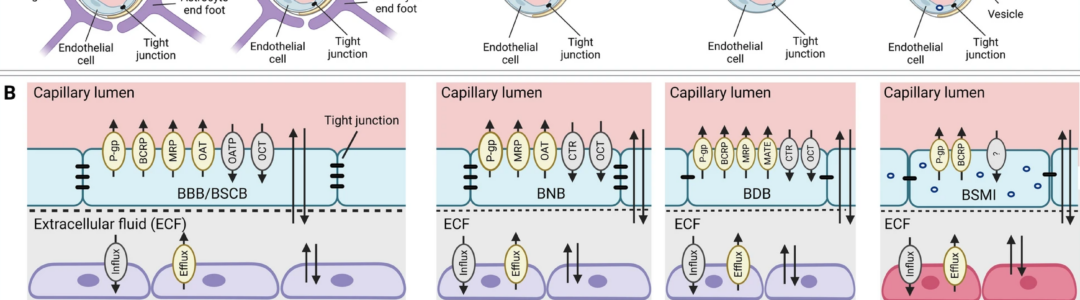
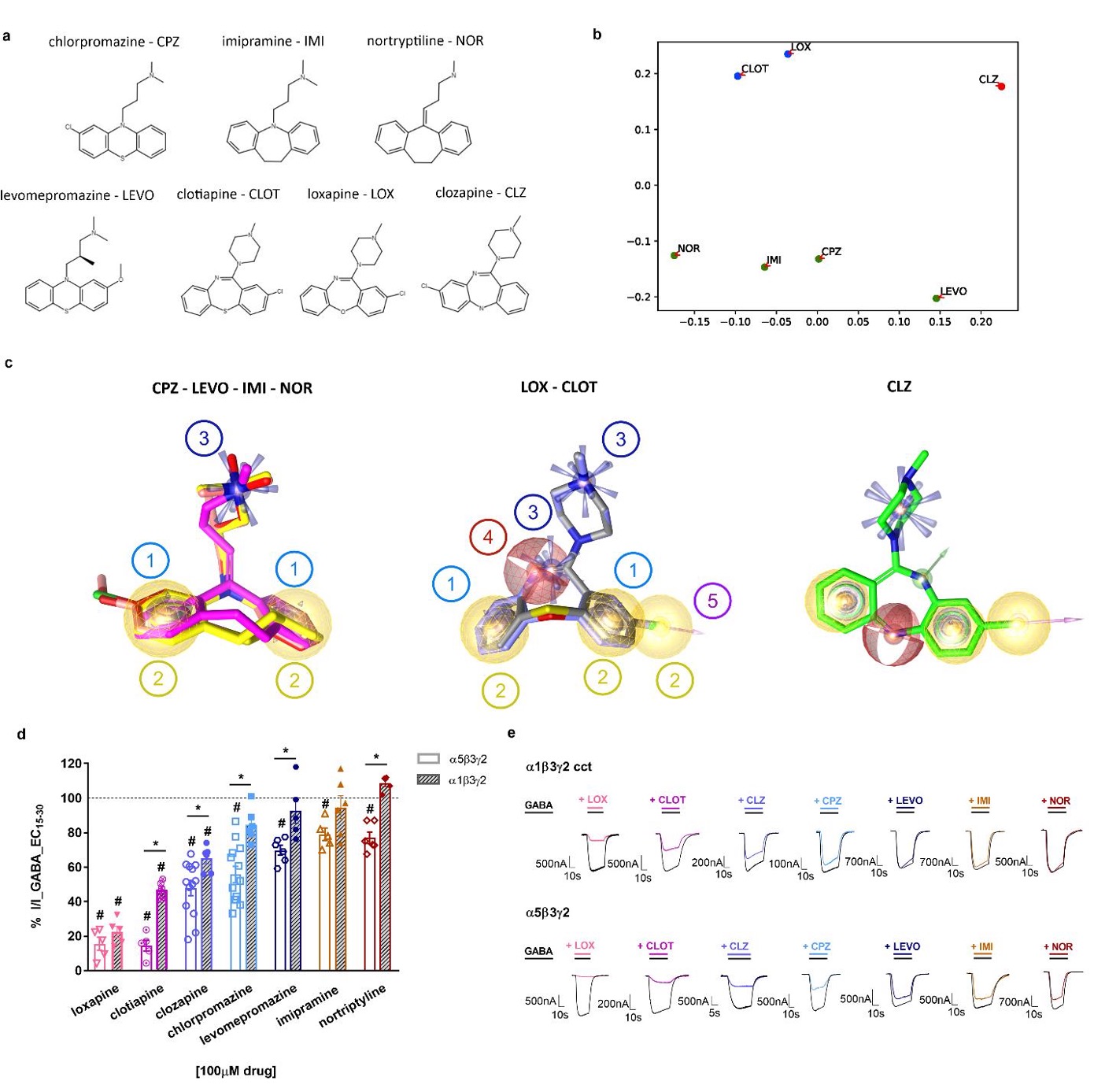
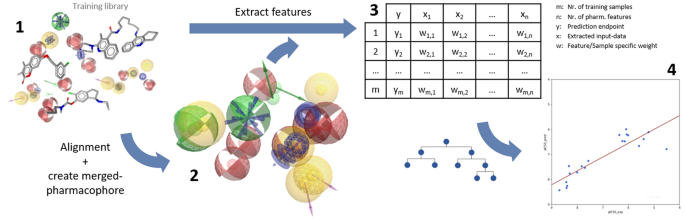
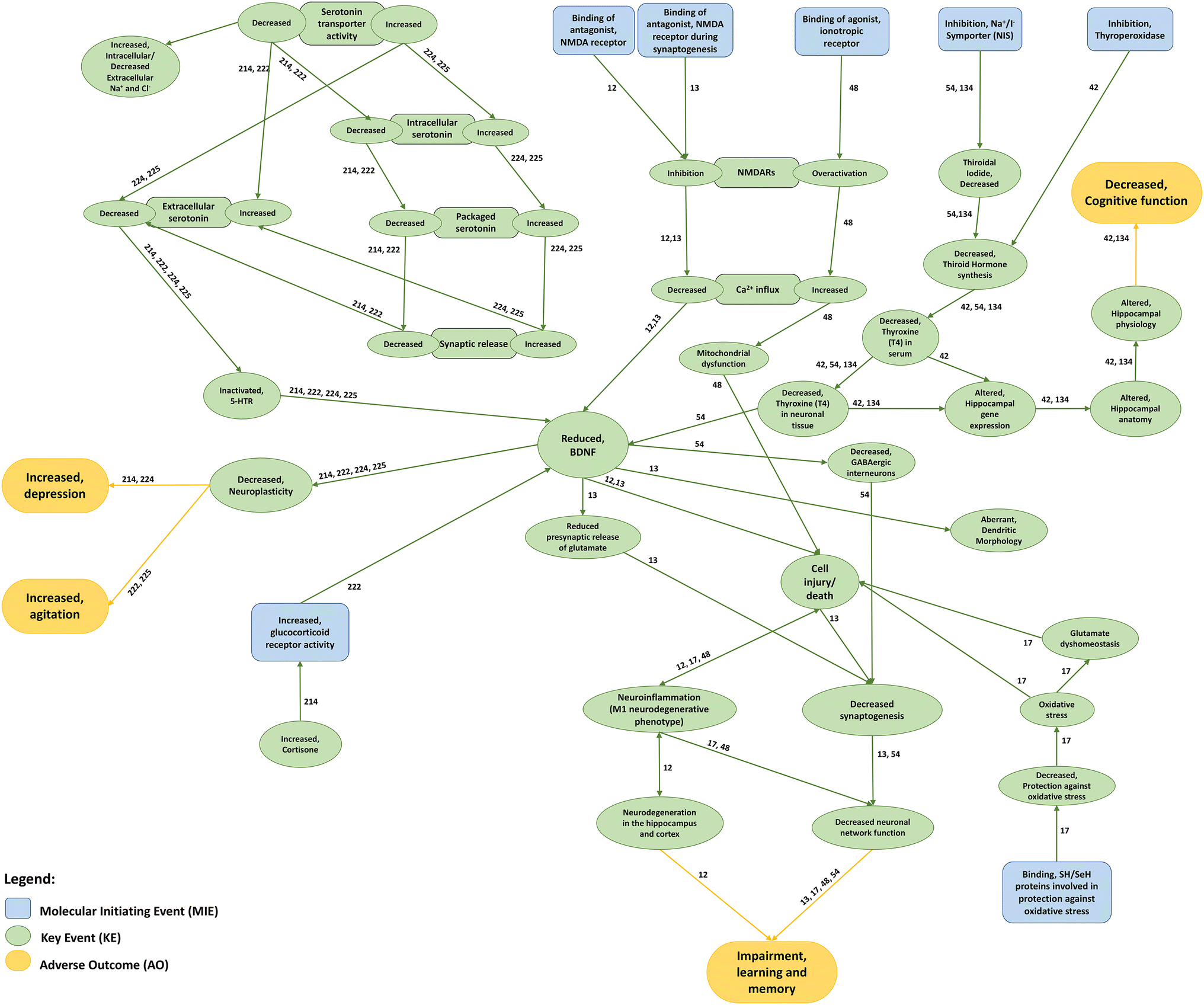
Chemotherapy-induced peripheral neuropathy (CIPN) represents a major unmet medical need that currently has no preventive and/or curative treatment. This is, among others, driven by a poor understanding of the contributive role of drug transport across biological barriers to target-site exposure.