Welcome to the NeuroDeRisk Project
NeuroDeRisk is an “Innovative Medicines Initiative” (IMI2) project aiming to provide novel validated integrated tools for improving the preclinical prediction of adverse effects of pharmaceuticals on the nervous system and thus help to de-risk drug candidates earlier in the Research and Development phases. The focus is on three major types of neurotoxicity:
SZ – Tox
convulsant & seizure-inducing effects
P/P – Tox
psychological & psychiatric side effects
PNS – Tox
peripheral nervous system toxicity
The adverse effects of pharmaceuticals on the central or peripheral nervous systems are poorly predicted by the current in vitro and in vivo preclinical studies performed during Research and Development (R&D) process. Therefore, increasing the predictivity of the preclinical toolbox is a clear need, and would benefit to human volunteers/patients (safer drugs) and Pharmaceutical Industry (reduced attrition). By combining top level scientists in neurobiology/toxicology with successful software developers, the NeuroDeRisk Consortium will aim at tackling three of the most challenging adverse effects: seizures, psychological/psychiatric changes, and peripheral neuropathies.
Our approach is a global one, starting with an in-depth evaluation of knowledge on mechanisms of neurotoxicity (biological pathways as well as chemical structures and descriptors, using in particular historical data), followed by the development of innovative tools, assays, and studies covering in silico, in vitro and in vivo approaches. This includes in particular:
- a molecular design platform,
- artificial intelligence,
- human induced pluripotent stem cells,
- blood-brain-barrier models,
- pharmacokinetics
- immunohistochemistry,
- transcriptomics,
- RNA editing biomarkers,
- video-monitoring and
- Nnon-invasive in vivo technologies (ultra high resolution video-monitoring, telemetry, actimetry)
The final step of our project aims at combining these tools in an integrated platform for improved risk-assessment and decision-points throughout the R&D process, providing a prediction platform for de-risking drug candidates based on potential neurotoxicity.
Latest News
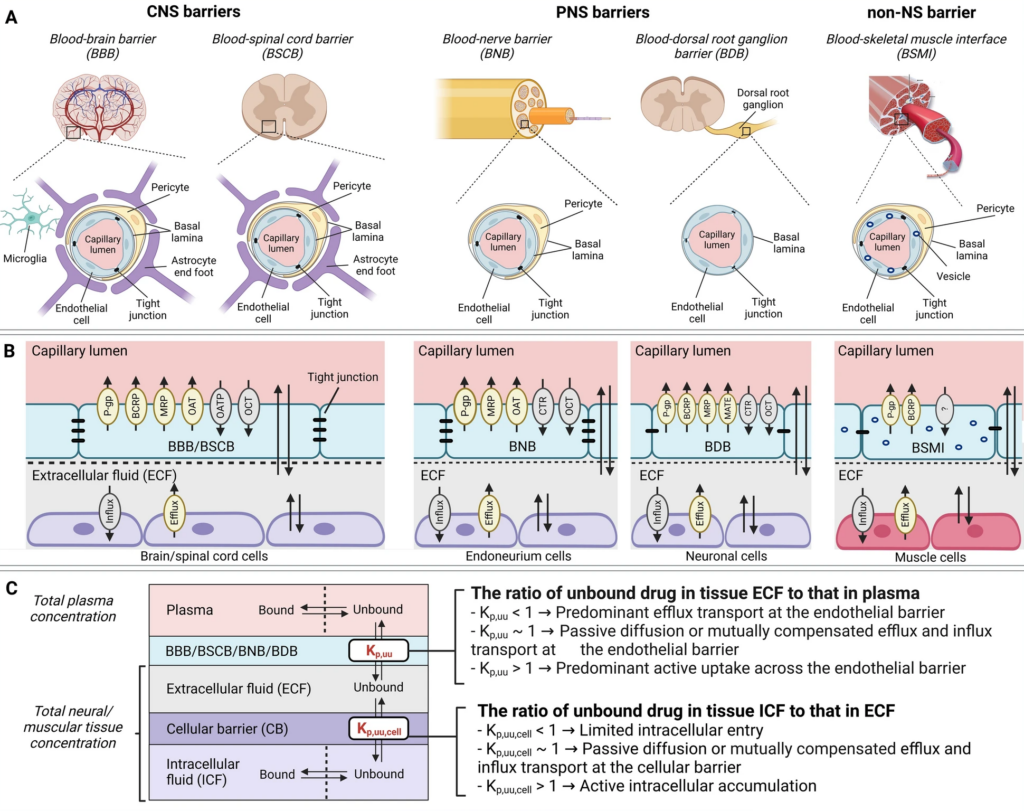
- Open Access Article: Lower the risk of chemotherapy-induced peripheral neuropathy (CIPN)Chemotherapy-induced peripheral neuropathy (CIPN) represents a major unmet medical need that currently has no preventive and/or curative treatment. This is, among others, driven by a poor understanding of the contributive role of drug transport across biological barriers to target-site exposure.
- Final NeuoDeRisk Project MeetingTime flies so fast. Time flies especially fast when you work together with such a fantastic team to improve the preclinical prediction of adverse effects of pharmaceuticals on the nervous system. With this meeting, our project comes to an end. One more reason to make this event something special.