Molecular basis of mood and cognitive adverse events elucidated via a combination of pharmacovigilance data mining and functional enrichment analysis.
Andronis, C., Silva, J.P., Lekka, E. et al.
Arch Toxicol (2020).
https://doi.org/10.1007/s00204-020-02788-1
Abstract
Drug-induced Mood- and Cognition-related adverse events (MCAEs) are often only detected during the clinical trial phases of drug development, or even after marketing, thus posing a major safety concern and a challenge for both pharmaceutical companies and clinicians. To fill some gaps in the understanding and elucidate potential biological mechanisms of action frequently associated with MCAEs, we present a unique workflow linking observational population data with the available knowledge at molecular, cellular, and psychopharmacology levels. It is based on statistical analysis of pharmacovigilance reports and subsequent signaling pathway analyses, followed by evidence-based expert manual curation of the outcomes. Our analysis: (a) ranked pharmaceuticals with high occurrence of such adverse events (AEs), based on disproportionality analysis of the FDA Adverse Event Reporting System (FAERS) database, and (b) identified 120 associated genes and common pathway nodes possibly underlying MCAEs. Nearly two-thirds of the identified genes were related to immune modulation, which supports the critical involvement of immune cells and their responses in the regulation of the central nervous system function. This finding also means that pharmaceuticals with a negligible central nervous system exposure may induce MCAEs through dysregulation of the peripheral immune system. Knowledge gained through this workflow unravels putative hallmark biological targets and mediators of drug-induced mood and cognitive disorders that need to be further assessed and validated in experimental models. Thereafter, they can be used to substantially improve in silico/in vitro/in vivo tools for predicting these adversities at a preclinical stage.
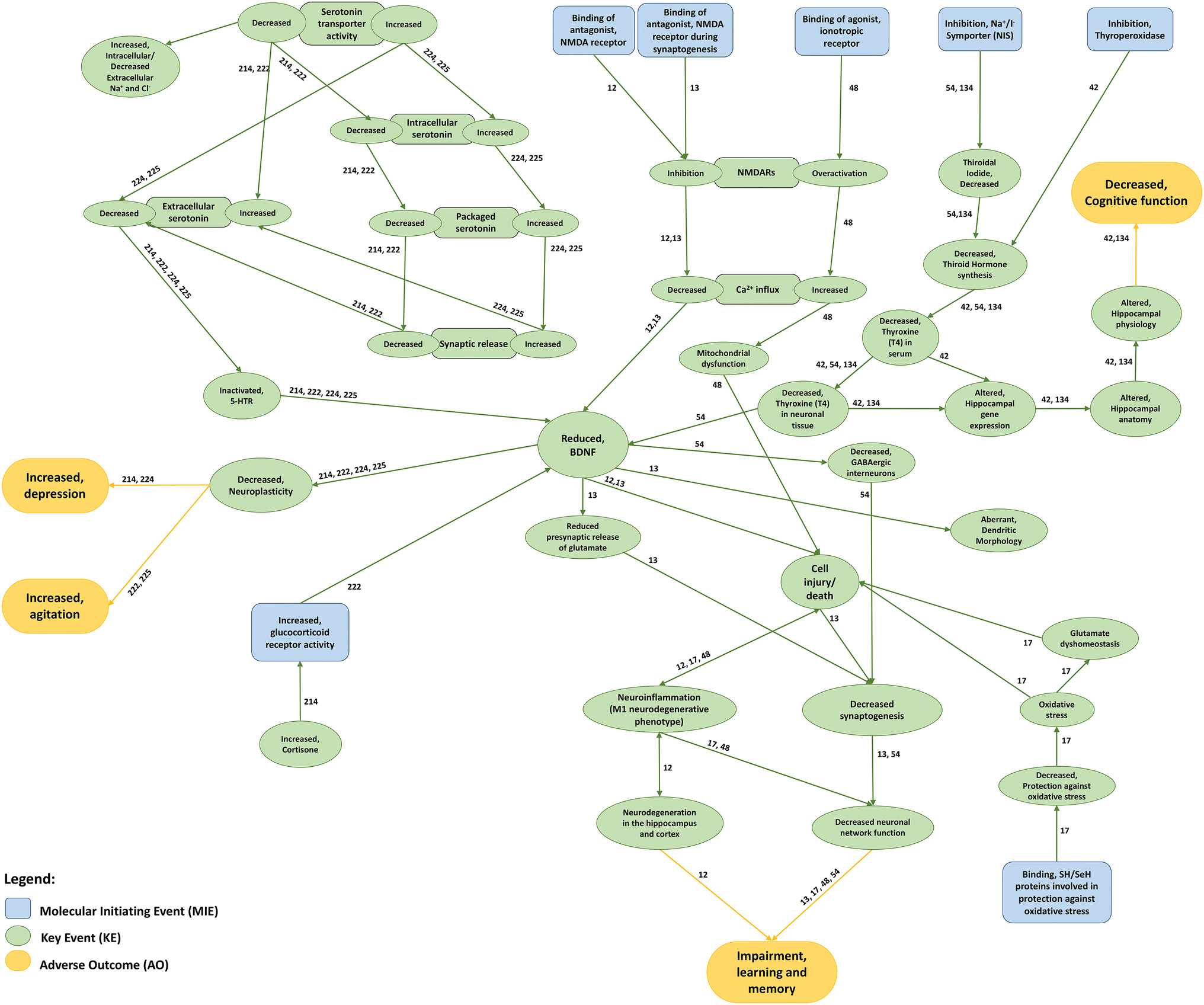
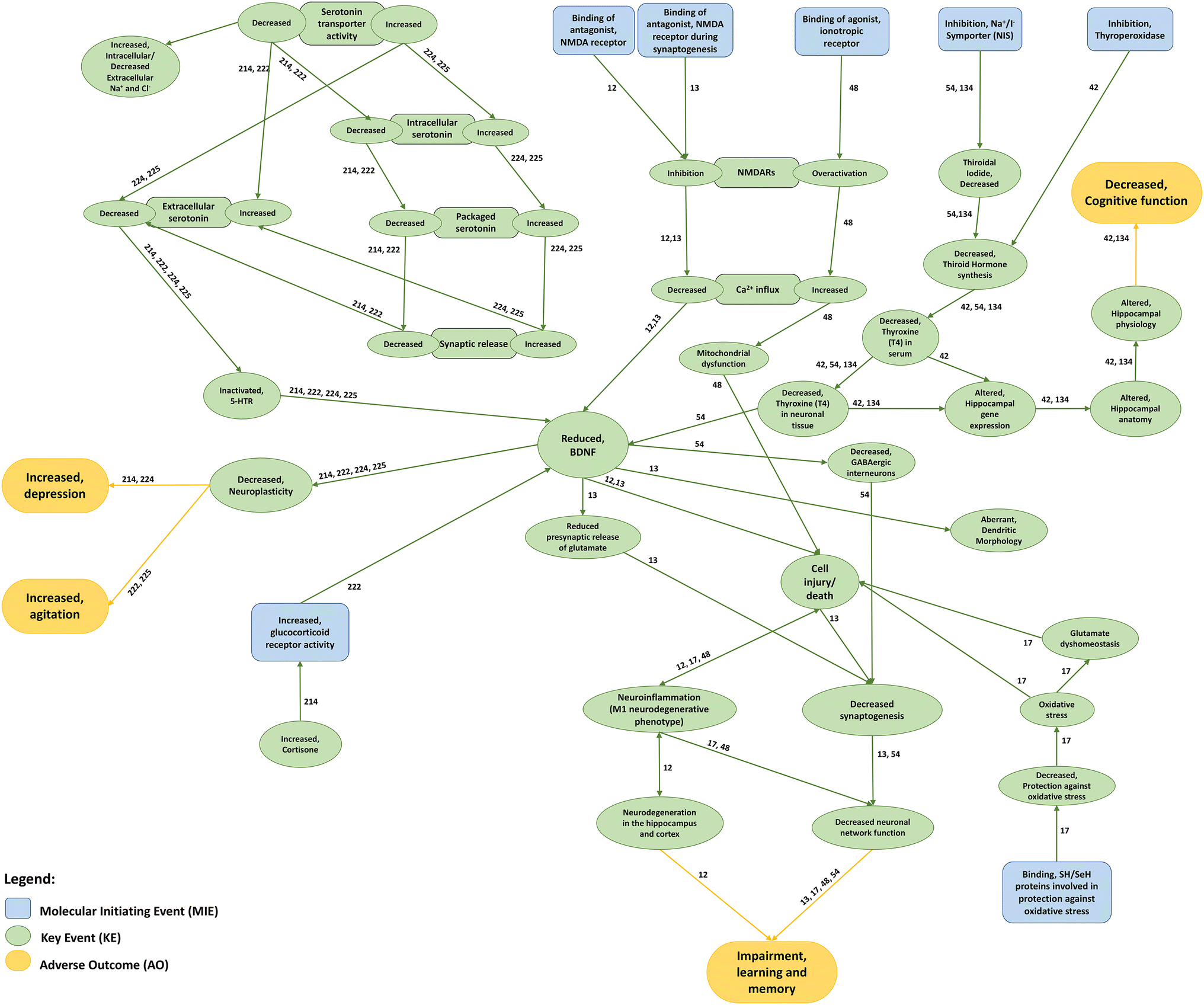
Interactions among AOPs associated with mood- and/or cognitive-related adverse outcomes. Molecular Initiating Events (blue boxes) trigger different downstream cascades of Key Events (green boxes) that ultimately lead to a mood- or cognitive-related Adverse Outcome (yellow boxes). Each AOP sequence of effects may be assessed by following its respective AOP ID next to each connecting edge. Information on MIEs, KEs and AOs herein depicted was retrieved from AOP databases (i.e. AOPWiki and AOP-KB)
Rights and Permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Keywords
Neurotoxicity, Pharmaceuticals’ safety, Psychiatric/psychological adverse events, Cross-talk analysis, Adverse outcome pathways